Home » Defective Medical Device Lawyers » CPAP Lawyers
Philips CPAP Lawyers
Lawyers Representing Victims of Recalled Philips Breathing Devices
Millions of people rely on devices manufactured by Philips Respironics to control medical conditions such as sleep apnea. Koninklijke Philips N.V., better known as Philips, is a global corporation headquartered in Amsterdam. Philips and its American subsidiary, Philips North America, manufacture products in numerous industries, including consumer goods, electronics, and healthcare.
On April 26, 2021, Philips announced potential health risks associated with certain products within its Sleep & Respiratory Care vision, specifically Continuous Positive Airway Pressure (CPAP) machines, Bi-Level Positive Airway Pressure (BiPAP) machines, and ventilators. On June 14, 2021, Philips formally issued a recall notice for specific devices manufactured between 2009 and April 26, 2021. Philips refers to itself as a “global leader in Sleep Diagnostic and Therapy Solutions” and is the largest manufacturer of CPAP devices in the world.

What Is Sleep Apnea?
Sleep Apnea is a serious medical condition involving abnormal breathing during sleep, where breathing stops and starts. Lapses in breathing during sleep can negatively affect the body’s oxygen supply. There are three types of sleep apnea:
- Obstructive Sleep Apnea – when the airway at the back of your throat becomes physically blocked, causing a temporary lapse in breathing
- Central Sleep Apnea – when an issue occurs with the brain’s system for controlling muscles involved in breathing and respiration
- Complex Sleep Apnea – when someone has obstructive sleep apnea and central sleep apnea at the same time
Symptoms of sleep apnea can include heavy snoring, gasping for air during sleep, insomnia, waking up with a dry mouth, and stopping breathing during sleep. If you experience any of these symptoms, you should consult with your doctor immediately. A common treatment for sleep apnea is your doctor prescribing a CPAP or BiPAP machine to use on a nightly basis.
2021 Recall of Philips CPAP Machines, BiPAP Machines, and Ventilators
On April 26, 2021, Philips announced potential health risks associated with their CPAP, BiPAP, and ventilator devices distributed between 2009 and April 26, 2021. Philips formally issued a recall notice for these devices on June 14, 2021. On June 30, 2021, the FDA issued an alert that certain Philips ventilators, CPAP, and BiPAP machines were recalled by Philips due to “potential health risks,” specifically that foam designed to reduce sound and vibration can break down and enter the device’s air pathway. On July 23, 2021, the FDA classified this recall as a Class 1 recall, the most serious classification available. A Class 1 recall means the use of that device could cause serious injury or death. If you were prescribed and used a CPAP, BiPAP, or ventilator device manufactured by Philips, you should consult with your doctor, as use of the device may cause medical issues. However, you should not stop using a prescribed device before consulting with a physician.
The recalled Philips devices contain polyester-based polyurethane (PE-PUR) sound abatement foam which is used to reduce sound and vibration in the devices. In these devices, the foam may break down and enter the air pathway of the device. If this happens, chemicals or black debris from the foam could be inhaled or swallowed by the user of the device. Exposure to the black debris or chemicals may cause serious injuries, including irritation, inflammation, headaches, asthma, adverse effects to organs (including liver and kidney), and even cancer. It is alleged that Philips knew of these dangers yet failed to take action or properly warn consumers.

Which Philips CPAP and BiPAP Devices Were Recalled?
To date, Philips has issued a recall for the following CPAP and BiPAP devices distributed from 2009 through April 26, 2021:
- E30
- DreamStation ASV
- DreamStation ST, AVAPS
- SystemOne ASV4
- C-Series ASV
- C-Series S/T and AVAPS
- OmniLab Advanced+
- SystemOne (Q-Series)
- DreamStation
- DreamStation Go
- Dorma 400
- Dorma 500
- REMstar SE Auto
Which Philips Ventilators Were Recalled?
Philips issued a recall for the following ventilators distributed from 2009 through April 26, 2021:
- Trilogy 100
- Trilogy 200
- Garbin Plus, Aeris, LifeVent
- A-Series BiPAP V30 Auto
- A-Series BiPAP A40
- A-Series BiPAP A30
Can a CPAP Machine Cause Health Problems?
Our medical device lawyers are currently investigating links between Philips CPAP, BiPAP, and ventilator machines and injuries such as cancer. The following injuries may be linked to inhaling polyurethane foam from an affected Philips device:
- Leukemia
- Breast cancer
- Lymphatic cancer
- Kidney cancer
- Liver cancer
- Nasal cancer
- Lung cancer or damage
- Non-Hodgkin’s lymphoma
- Brain cancer
- Liver cancer
- Multiple myeloma
- Prostate cancer
- Bladder cancer
- Testicular cancer
- Stomach cancer
- Hematopoietic cancer
- Papillary carcinoma and other thyroid cancers
- Chronic bronchitis
- Respiratory failure
- Asthma
- Heart failure or heart attack
- Stroke
- Irritation of ear, nose, or throat
- Nausea
- Liver disease or damage
If you use a Philips CPAP machine, BiPAP machine, APAP machine, or ventilator and have experienced any of the above symptoms or been diagnosed with any of these conditions, you should consult your doctor immediately. Philips has sent users of affected devices a letter explaining the device is subject to a recall and that further use could cause risk of serious illness, disease, or death. Our Philips CPAP recall lawyers are available to speak with you about your legal rights and options.
Philips CPAP Lawsuits
Nationwide, lawsuits are being filed by injured victims against Philips and Philips North America for injuries caused by the recalled devices. The lawsuits allege Philips has known about the risks of their sound abatement foam for years but failed to properly warn consumers or the public. Further, lawsuits filed by attorneys representing victims of CPAP machines have alleged Philips negligently and defectively designed their products, knowingly sold defective devices harmful to consumers, and failed to warn consumers of the risks of polyester-based polyurethane (PE-PUR) sound abatement foam. Under product liability laws, a medical device manufacturer can be held liable for the negligent design of a device, and where side effects are either known or should be known but not disclosed, negligently failing to warn consumers and patients.
Users of the Philips machines affected by the degradation of the foam have reported experiencing pain and discomfort, which could entitle them to monetary damages. Additionally, these individuals have other medical conditions that have been worsened by the continued use of the CPAP machines. The possibility of suffering further harm due to a product that was supposed to help can be devastating. An experienced lawyer can assess your situation, explain your legal rights, and help you pursue justice. Those injured by a Philips CPAP machine may be able to recover compensation for medical bills, lost wages, pain and suffering, and more. If the Philips device caused a loved one to pass away, a wrongful death lawsuit may be filed.
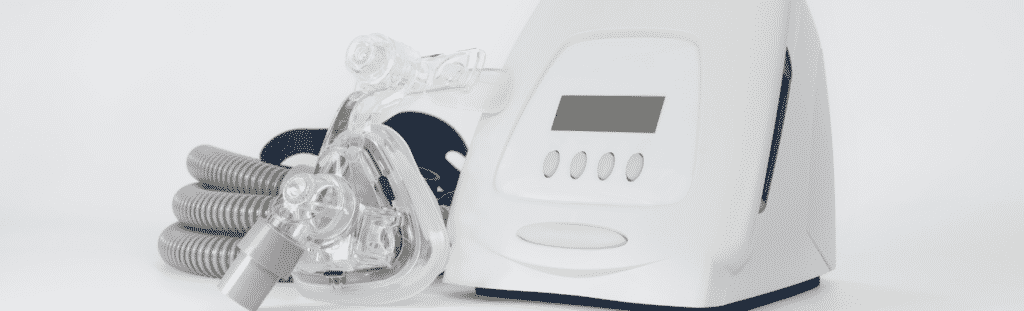
Did You Suffer Injuries Caused by a Philips CPAP Machine or Breathing Device?
If you or your loved one used a Philips CPAP, BiPAP or mechanical ventilator machine and has been affected by the recall, you may be eligible to recover compensation for medical bills, lost wages, pain and suffering, and other damages. For a free initial consultation with a CPAP injury lawyer today, call NST Law at 800-529-4004 or contact us online. NST Law represents people nationwide in product liability, mass tort, medical device, and pharmaceutical cases, and our team has recovered over $1.5 billion in compensation for clients over the last 30+ years. By combining our experience, resources, and personalized client service, we are Champions for the Injured.
Disclaimer: This is a legal advertisement. Do not stop using a prescribed medical device without first consulting with your doctor. Discontinuing a prescribed medical device without your doctor’s advice can result in injury or death.
The choice of a lawyer is very important and should not be based solely upon advertisements. Free background information available upon request. Nahon, Saharovich and Trotz is a large personal injury law firm. Various firm attorneys will provide legal services, depending upon the particular state, although cases may be referred to other co-counsel. Prior results do not guarantee similar outcomes.
